Impact of metabolism and temperature on 2H/1H fractionation in lipids of the marine bacterium Shewanella piezotolerans WP3
Compound-specific hydrogen isotopes have increasingly been used as a powerful proxy for investigating biogeochemical cycles and climate change over the past 2 decades. Understanding the hydrogen isotope in extant organisms is fundamental for us to interpret such isotope signals preserved in natural environmental samples. Here, we studied the controls on hydrogen isotope fractionation between fatty acids and growth water by an Fe-reducing heterotrophic marine bacterium Shewanella piezotolerans WP3 growing on different organic substrates, including N-acetyl-D-glucosamine (GlcNac), glucose, acetate, pyruvate, L-alanine, and L-glutamate. Meanwhile, we also evaluated the impact of growth temperature on the hydrogen isotope composition of fatty acids using GlcNac as the sole organic substrate. Our results show that the abundance-weighted mean fatty-acid / water fractionations () display considerable variations for cultures grown on different substrates. Specifically, WP3 yielded the most 2H-enriched fatty acids growing on L-glutamate and pyruvate with an of 52 ± 14 ‰ and 44 ± 4 ‰, respectively, and exhibited 2H depletion using GlcNac (−76 ± 1 ‰) and glucose (−67 ± 35 ‰) as sole carbon sources and relatively small fractionations on acetate (23 ± 3 ‰) and L-alanine (−4 ± 9 ‰). Combined with metabolic model analysis, our results indicate that the central metabolic pathways exert a fundamental effect on the hydrogen isotope composition of fatty acids in heterotrophs. Temperature also has an obvious influence on the δ2H values of fatty acids, with strong 2H depletion at an optimal growth temperature (−23 ± 2 ‰ and −23 ‰ growing at 15 and 20 ∘C, respectively) and relatively small fractionations at non-optimal temperatures (4 ± 5 ‰, −4 ± 12 ‰, and 15 ± 41 ‰ at 4, 10, and 25 ∘C, respectively). We hypothesized that this may be associated with temperature-induced enzyme activity for nicotinamide adenine dinucleotide phosphate (NADPH) production. This study helps understand the controlling factors of hydrogen isotope fractionation by marine bacteria, laying the foundation for further interpreting the hydrogen isotope signatures of lipids as an important proxy to decode the biogeochemical cycles and ecological changes in marine sediments.
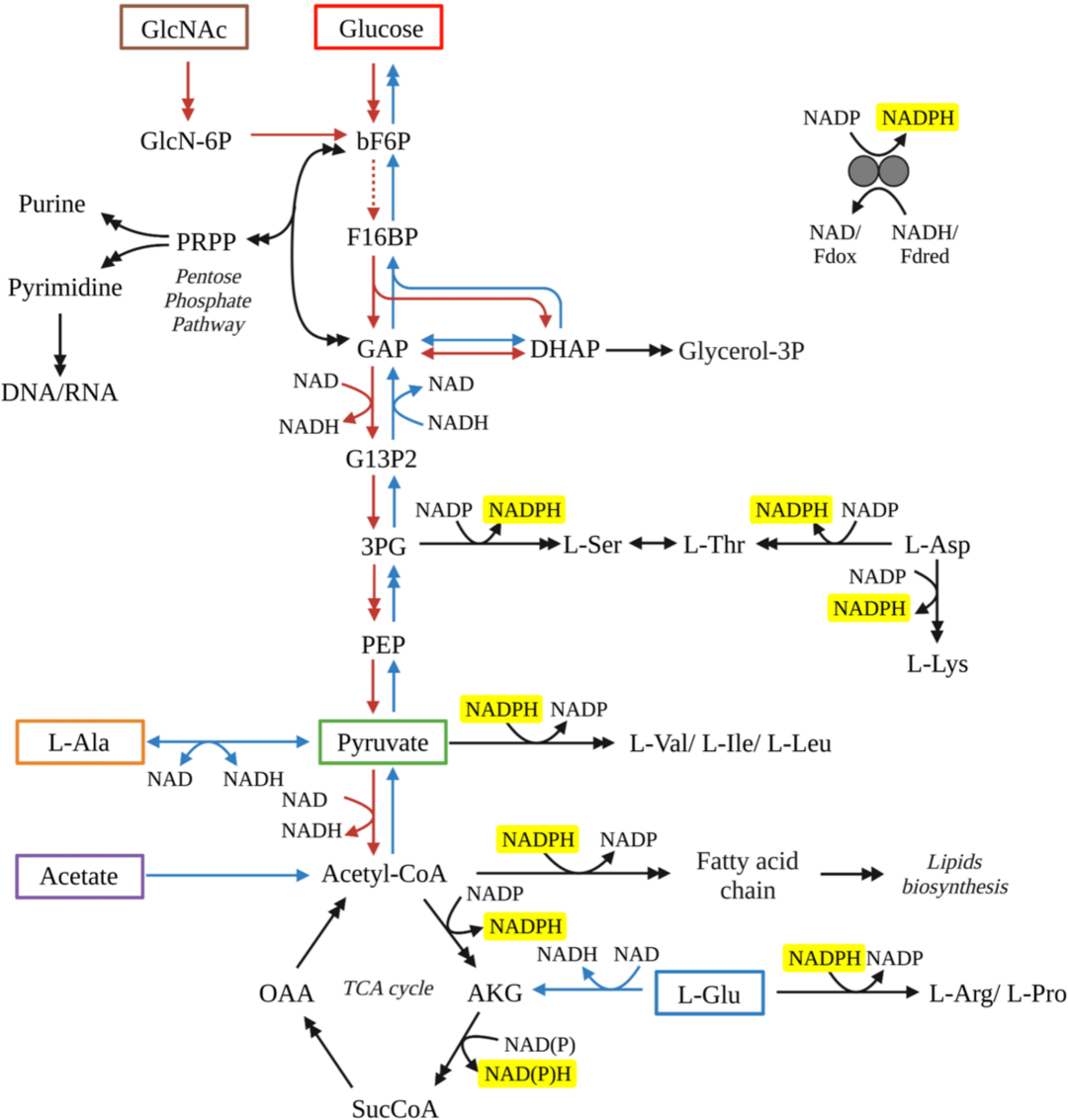
Figure Schematic diagram of the metabolic pathways for WP3 by utilizing various substrates. Tested substrates in this study include GlcNac (brown box), glucose (red box), acetate (purple box), pyruvate (green box), L-alanine (orange box) and L-glutamate (blue box). Red arrows represent reactions through glycolysis pathways, while blue arrows represent reactions through glycogenesis pathway. Black arrows represent reactions required no matter which substrate was used. Double arrows indicate that multiple reactions are involved in the conversion between two compounds shown. All reactions with NADPH as a cofactor have been highlighted with yellow background. Abbreviations: 3PG, 3-phospho-D-glycerate; Akg, 2-oxoglutarate; bF6P, beta-D-fructose 6-phosphate; DHAP, dihydroxyacetone phosphate; F16BP, beta-D-fructose 1,6-bisphosphate; G13P2, D-glycerate 1,3-diphosphate; G6P, D-glucose 6-phosphate; GAP, glyceraldehyde 3-phosphate; GlcN-6P, D-glucosamine 6-phosphate; GlcNAc, N-acetyl-D-glucosamine; Glycerol-3P, glycerol 3-phosphate; OAA, oxaloacetate; PEP, phosphoenolpyruvate; Pi, phosphate; PPi, diphosphate; SucCoA, succinate CoA.
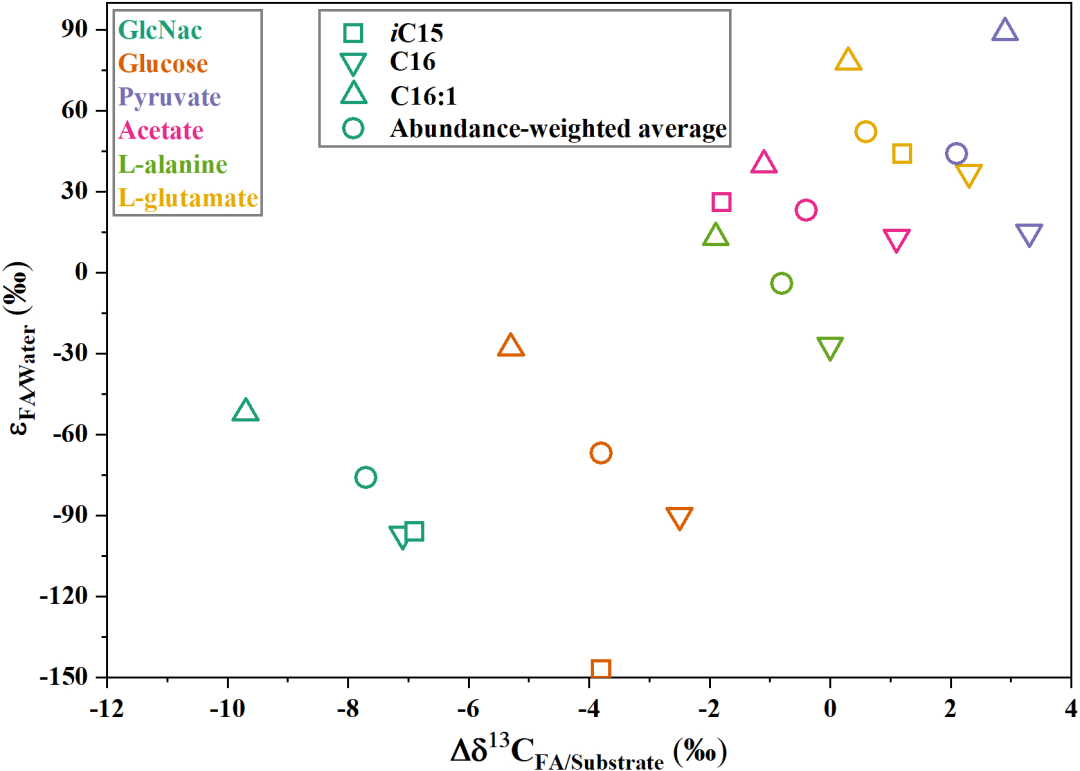
Figure 6 The values of carbon and hydrogen fractionation of fatty acids in S. piezotolerans WP3 growing on different organic substrates. The values are cited from Chen et al. (2022).


 loading......
loading......